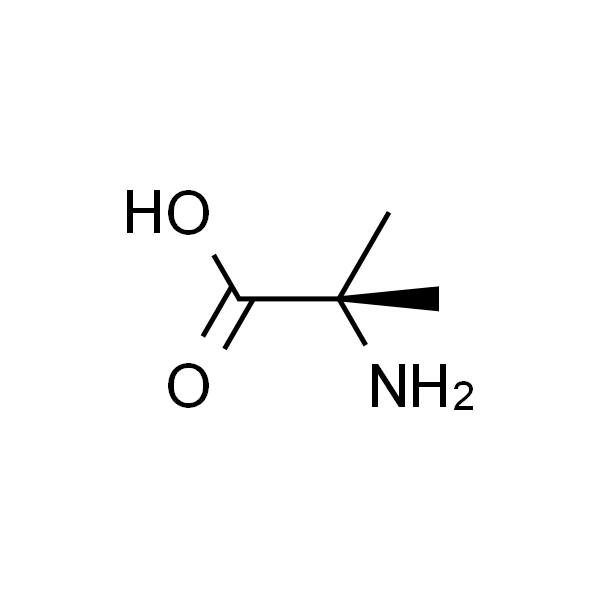
2-甲基丙氨酸
| EC | EINECS 200-544-0 |
| MDL | MFCD00008049 |
| 描述 | 是一种稀有的、非蛋白氨基酸,是嘧啶代谢的终产物,随着尿液排出,在一些真菌来源的抗生素中有发现。 |
| InChIKey | FUOOLUPWFVMBKG-UHFFFAOYSA-N |
| InChI | InChI=1S/C4H9NO2/c1-4(2,5)3(6)7/h5H2,1-2H3,(H,6,7) |
| PubChem CID | 6119 |
| 别名 | 2-氨基异酪酸 |
| 英文名称 | 2-Aminoisobutyric Acid |
| CAS | 62-57-7 |
| 分子式 | C4H9NO2 |
| 分子量 | 103.12 |
| 纯度 | ≥95% |
| 单位 | 瓶 |
| 生物活性 | NSC 16590 inhibits the production of endogenous ethylene in the cotyledonary segments of cocklebur.[1] |
| In Vitro | NSC 16590 (α-Aminoisobutyric acid , AIB) inhibits the production of endogenous ethylene in the cotyledonary segments of cocklebur (Xanthium pennsylvanicum Wallr.) seeds most strongly. NSC 16590 at 4 mM inhibits the formation of ethylene by about 50%, although the O2 uptake of the segments is not affected even at 20 mM. NSC 16590 also inhibits ethylene formation in the stem segments of etiolated pea (Pisum satiuum L. cv. Alaska) seedlings. Kinetic analysis with cell free extracts from etiolated pea shoots reveals that NSC 16590 competitively inhibits the conversion of ACC into ethylene[1]. |
| SMILES | NC(C)(C)C(O)=O |
| 靶点 | Others |
| 数据来源文献 | [1]. Shigeru Satoh, et al. α-Aminoisobutyric acid: A probable competitive inhibitor of conversion of 1-aminocyclopropane-1-carboxylic acid to ethylene. Plant and Cell Physiology, Volume 21, Issue 6, 1 September 1980, Pages 939-949. |
| 规格 | 50mg 100mg 250mg 500mg |
是一种化合物,具有生物或化学活性。