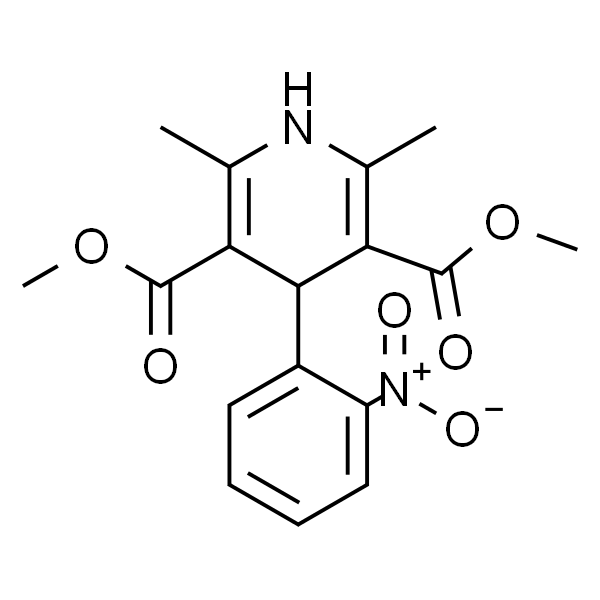
硝苯地平

| MDL | MFCD00057326 |
| EC | EINECS 244-598-3 |
| 别名 | 利心平;硝苯吡啶 |
| 英文名称 | Nifedipine |
| CAS | 21829-25-4 |
| 分子式 | C17H18N2O6 |
| 分子量 | 346.33 |
| 纯度 | HPLC≥98% |
| 单位 | 瓶 |
| 生物活性 | Nifedipine 是有效的钙离子通道 (calcium channel) 阻滞剂,常用于治疗心肌功能不全。[1-4] |
| In Vitro | 硝苯地平(100μM)显著降低WKPT-0293 Cl.2细胞的活力,硝苯地平(10或100μM)加FAC的处理诱导细胞活力显著降低,但对照之间的生存力没有显著差异细胞和用100μMFAC或1和10μM硝苯地平处理的细胞。硝苯地平(1,10或100μM)显著增加WKPT-0293 Cl.2细胞中的铁水平。硝苯地平治疗还增加WKPT-0293 Cl.2细胞中TfR1,DMT1 + IRE和DMT1-IRE的表达。此外,与硝苯地平(100μM)和FAC(100μM)共同处理可增加WKPT-0293 Cl.2细胞中的TfR1,DMT1 + IRE和DMT1-IRE表达[2]。在中等浓度范围内,硝苯地平和利托君对单独使用每种药物产生的收缩性抑制作用明显更大。硝苯地平加硝酸甘油或硝苯地平加阿托西班的组合比单独的硝酸甘油或阿托西班产生显著更大的抑制作用但不大于硝苯地平。硝苯地平和NS-1619(Ca2 +激活的K + [BKCa]通道开放剂)的组合降低了每种药物的抑制作用[3]。硝苯地平(2μM)显著抑制P. capsici菌丝体生长和孢子形成。硝苯地平诱导的菌丝生长抑制是钙依赖性的。硝苯地平(0.5μM)以钙依赖性方式增加P. capsici对H2O2的敏感性。硝苯地平抑制P. capsici的毒力和参与致病性的基因表达[4]。 |
| In Vivo | 在硝苯地平(50 mg / kg)和CsA治疗的大鼠中,BL尺寸(BLi和BLk),MD尺寸(MDk)和垂直尺寸(VHi和VHk)在结束时显着增加(P <0.05)。第4周[1]。 |
| SMILES | O=C(C1=C(C)NC(C)=C(C(OC)=O)C1C2=CC=CC=C2[N+]([O-])=O)OC |
| 靶点 | Calcium Channel |
| 动物实验 | 所有30只大鼠随机分成三组,每组10只。第1组(对照组)接受橄榄油8周。第2组和第3组在橄榄油中接受CsA(30mg/kg体重)和Nf(50mg/kg体重)的组合,持续8周。在第3组中,在第5周,将Azi(10mg/kg体重)加入该方案中。总研究期为8周。 |
| 细胞实验 | 使用MTT测定评估细胞活力。简而言之,在37℃下孵育4小时之前,向每个孔中加入总共25μLMTT(在PBS中1g/L)。通过加入100μL裂解缓冲液(20%SDS的50%N’-二甲基甲酰胺,pH4.7)终止测定。通过使用ELX-800微孔板测定读数器在570nm波长下测量光密度(OD),结果表示为在对照细胞中测量的吸光度的百分比。 |
| 数据来源文献 | [1]. Ratre MS, et al. Effect of azithromycin on gingival overgrowth induced by cyclosporine A + nifedipine combination therapy: A morphometric analysis in rats. J Indian Soc Periodontol. 2016 Jul-Aug;20(4):396-401.
[2]. Yu SS, et al. Nifedipine Increases Iron Content in WKPT-0293 Cl.2 Cells via Up-Regulating Iron Influx Proteins. Front Pharmacol. 2017 Feb 13;8:60 [3]. Carvajal JA, et al. The Synergic In Vitro Tocolytic Effect of Nifedipine Plus Ritodrine on Human Myometrial Contractility. Reprod Sci. 2017 Apr;24(4):635-640. [4]. Liu P, et al. The L-type Ca(2+) Channel Blocker Nifedipine Inhibits Mycelial Growth, Sporulation, and Virulence of Phytophthora capsici. Front Microbiol. 2016 Aug 4;7:1236 |
| 规格 | 50mg 100mg 10mM*1mL (in DMSO) 500mg |
是有效的钙离子通道 (calcium channel) 阻滞剂。